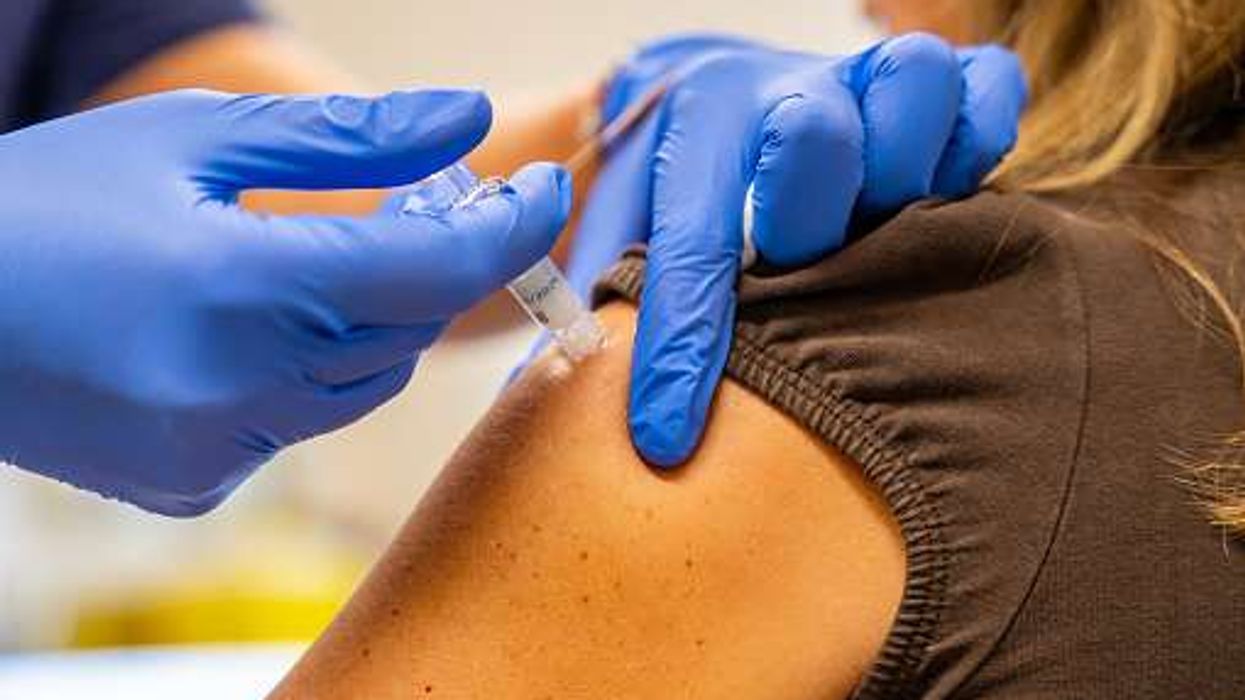
The Medicines and Healthcare Products Regulatory Agency (MHRA) has approved the zapomeran (Kostaive) mRNA COVID-19 vaccine.
The drug has been approved under the International Recognition Procedure (IRP), and the Reference Regulator was the European Medicines Agency (EMA).
It is a single 0.5 ml booster jab intended for individuals aged 18 or above, and the dose must be administered via injection in the upper arm.
The vaccine contains self-amplifying messenger RNA (sa-mRNA) that helps the body's cells produce the SARS-CoV-2 spike protein for a short duration, preparing the immune system to resist the virus.
Some of the common side effects include pain or tenderness at the injection site, tiredness, chills, fever, muscle and joint ache, headache, and dizziness, which will disappear in a few days.
The MHRA has urged people experiencing side effects to seek expert medical help.
“The approval of zapomeran (Kostaive) provides an alternative vaccine for use in adults to prevent COVID-19 caused by SARS-CoV-2,” said Julian Beach, interim executive director of Healthcare Quality and Access at the MHRA.
He also assures that all the medications approved by the MHRA will still be under their close supervision to ensure safety and improved effectiveness.