Key Summary
- Firm still selling illegal trial drug replicas online
- MHRA seized thousands of unlicensed injections in October
- Experts warn of safety risks, especially in winter
The company associated with the sale of counterfeit versions of a weight-loss medicine continues to be in business, despite a Medicines and Healthcare products Regulatory Agency (MHRA) raid in October.
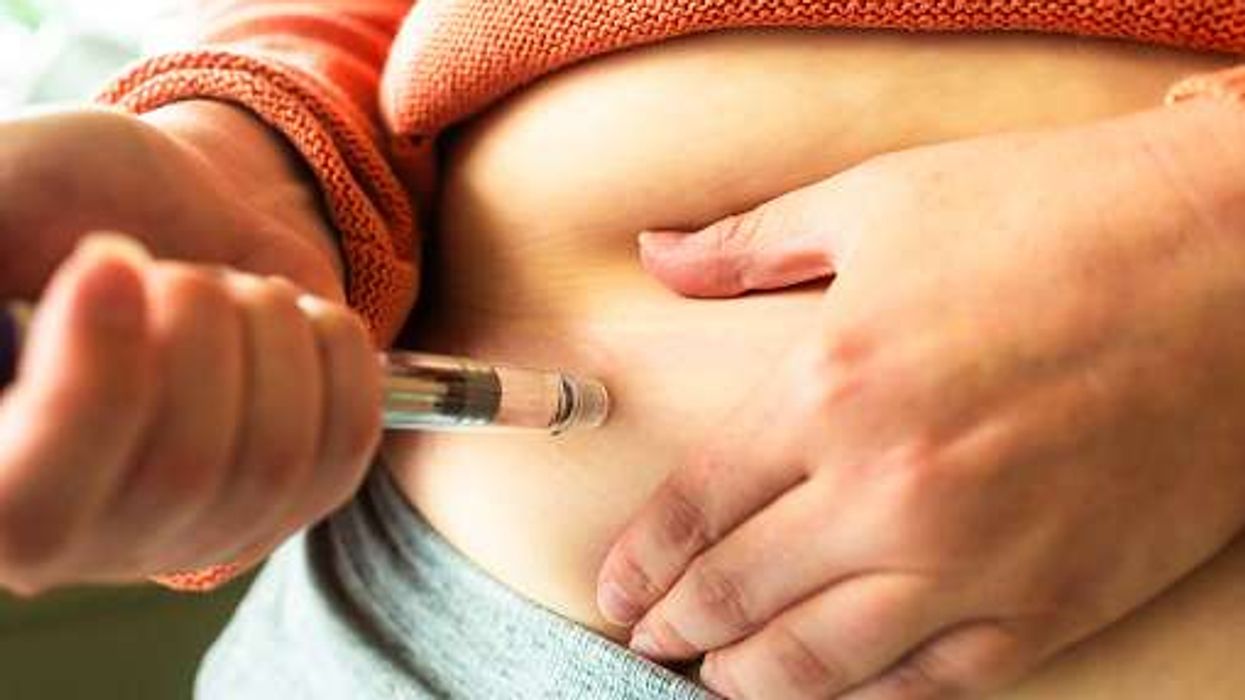
The company, Alluvi Healthcare Limited, is still selling replicas of retatrutide, The Guardian reports.
Retatrutide, an injection developed by the US drugmaker Eli Lilly, is still undergoing clinical trials.
Hence its sale is illegal, and they are still unapproved for use.
But the accused is selling its replicas through strong social media promotions, the daily added.
During the October raid, MHRA officers had confiscated tens of thousands of empty pens ready to be filled, raw chemical ingredients, and more than 2,000 unlicensed retatrutide and tirzepatide branded Alluvi pens.
Despite this, Alluvi continues to sell its jabs on multiple Telegram channels with thousands of subscribers.
They continue to offer “retatrutide 40mg x2 Bundle (R&D Only)” for £339.99.
A Channel 4 investigation had earlier found that those who spoke against Alluvi found that their social media accounts were taken over.
Jason Murphy, a weight-loss expert and head of pharmacy at Chemist4U warned of potential complications these drugs undergo during winter.
“These drugs are made from living organisms and are highly sensitive to temperature variations and extreme highs or lows,” he said.