Key Summary
- MHRA restricts IXCHIQ to healthy adults aged 18–59 only.
- Not for over-60s or people with chronic conditions or weakened immunity.
- Vaccinate at least 30 days before travel for safety monitoring.
The Medicines and Healthcare products Regulatory Agency (MHRA) has barred the use of Chikungunya vaccine, IXCHIQ, to those aged 60 and above, and those having high blood pressure, heart disease, diabetes, or chronic kidney disease, irrespective of their age.
The regulator announced these changes following recommendations by the Commission on Human Medicines (CHM), the government’s independent expert advisory committee.
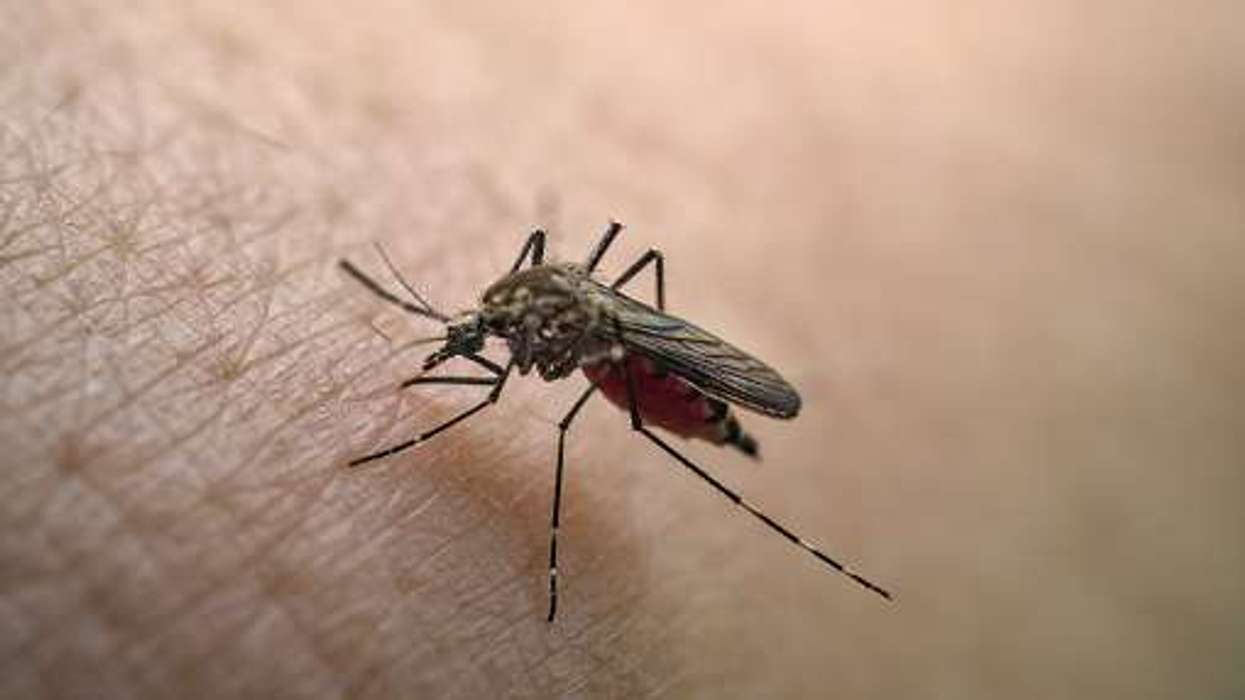
Chikungunya virus (CHIKV) is a mosquito-borne virus found in the subtropical parts of the Americas, Africa, Southeast Asia, India and Pacific regions with symptoms like sudden fever, joint pain (arthralgia), headache, muscle pain, join swelling or rashes.
IXCHIQ vaccine should also not be used in individuals who are immunodeficient or immunosuppressed, including IgA (immunoglobulin A) deficiency, and those who have a history of thymus disorder and/or thymectomy.
The commission concluded that the vaccine is beneficial only for those aged 18–59 years and do not suffer from the above conditions.
Before administering the vaccine, trained healthcare professionals must undertake a comprehensive benefit risk assessment.
The vaccine should be taken at least 30 days before travel to ensure that if any serious adverse reactions occurs, the individual is still in the UK with appropriate access to healthcare.