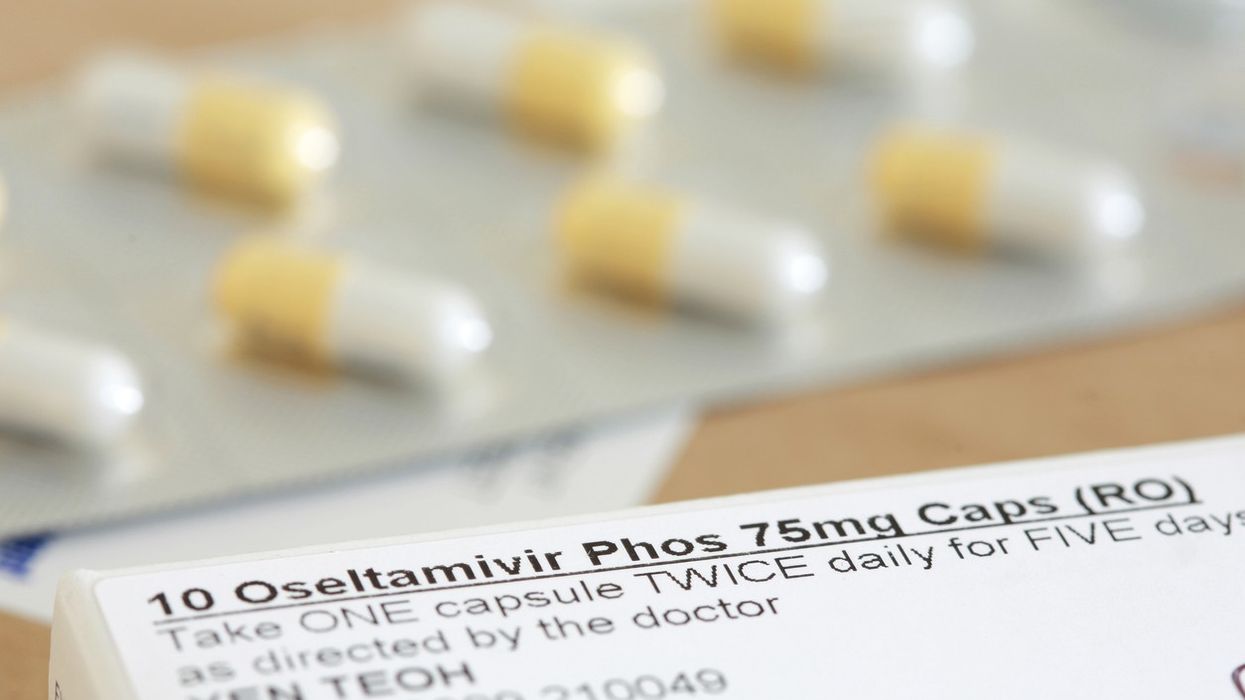
Adults are advised to start taking oseltamivir or zanamivir within 48 hours of onset of flu symptoms.
As flu cases increases in the community, the Department of Health and Social Care (DHSC) has allowed pharmacists to supply two antiviral medicines (oseltamivir and zanamivir) at NHS expense to help treat and prevent influenza in “at-risk” patients.
Prescribers working in primary care can also prescribe the medicines for patients in clinical at-risk groups as well as those who are at risk of severe illness and/or complications from flu if not treated.
This is in accordance with National Institute for Health and Care Excellence (NICE) guidance, and Schedule 2 to the National Health Service (General Medical Services Contracts (Prescription of drugs etc) Regulations 2004), commonly known as the Grey List or Selected List Scheme (SLS), the DHSC said in its letter issued on 14 December via its Central Alerting System (CAS).
Meanwhile, Community Pharmacy England (CPE) has reminded pharmacy contractors who receive FP10 NHS prescriptions, written generically or by brand, for oseltamivir (Tamiflu) or zanamivir (Relenza), the antivirals included in the SLS list, that prescriptions for these products must be endorsed SLS by the prescriber.
If the SLS endorsement is missing, the prescription should not be dispensed and will not be passed for payment by NHS Prescription Services, and pharmacy staff cannot make the SLS endorsement themselves.
Citing UKHSA surveillance data, the health department has warned that influenza is circulating in the community and has advised use of antiviral for the prevention and treatment.
The letter from Professor Chris Whitty, Chief Medical Officer for England, and David Webb FRPharmS, Chief Pharmaceutical Officer for England, has also highlighted that adults should start taking these antivirals within 48 hours of onset of flu symptoms.
For children who are five years old or over, treatment with zanamivir should begin within 36 hours of onset of symptoms
Children over 12 months and adults who are not able to swallow capsules can be prescribed oral oseltamivir suspension, they stated.
Oseltamivir can be prescribed in children under 12 months, including full term newborns who present with symptoms typical of influenza, and efficacy has been demonstrated when treatment is initiated within two days of first onset of symptoms.
The DHSC letter reads: “It is important that pharmacists ensure antiviral medicines are issued to patients promptly. If unable to fulfil the whole prescription, you should consider how best to assist patients gain timely access to antivirals. e.g. whether other community pharmacies locally have stock. If they do, either arrange for the patient to collect the stock from that pharmacy or get the stock transferred to your pharmacy.”
The officers have also advised all health and social care workers to get vaccinated and observe appropriate infection control measures to protect their patients from influenza.