The government has made a series of commitments, including removing racial bias from data sets used in clinical studies.
Reacting to recommendations from a UK-first independent review, the Department of Health and Social Care (DHSC) has outlined action to tackle potential bias in the design and use of medical devices.
Professor Dame Margaret Whitehead, professor of public health at the University of Liverpool, was appointed to lead the review, which focused on three areas – optical devices such as pulse oximeters, AI-enabled devices, and polygenic risk scores (PRS) in genomics.
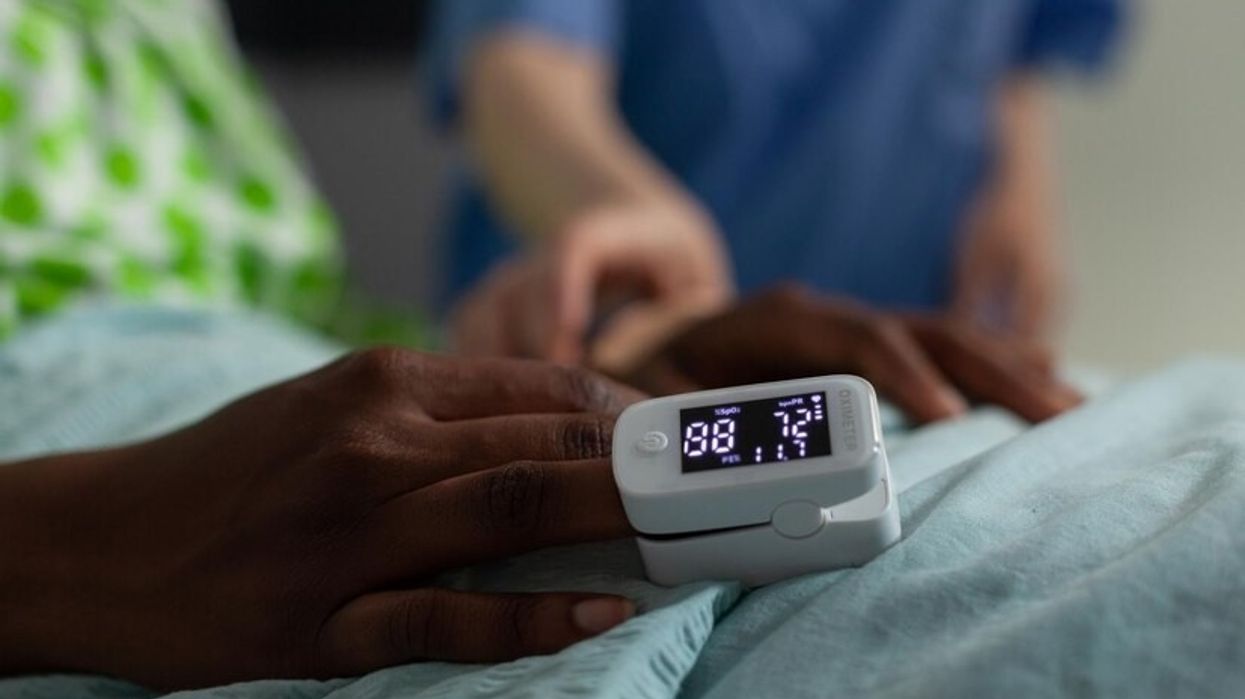
The DHSC commissioned the medical devices review after concerns were raised that pulse oximeters – widely used during the COVID-19 pandemic to monitor blood oxygen levels – were not as accurate for patients with darker skin tones. There were worries that this could cause delays in treatment if dangerously low oxygen levels in such patients were missed.
However, no evidence was found from NHS studies indicating that this differing performance had an impact on patient care.
Accepting the report’s conclusions, the DHSC has committed to several actions, such as ensuring the safe use of pulse oximeter devices across a range of skin tones within the NHS and eliminating racial bias from data sets employed in clinical studies.
Andrew Stephenson, the minister of state for health and social care, thanked Professor Whitehead for carrying out this important review.
He said: “Making sure the healthcare system works for everyone, regardless of ethnicity, is paramount to our values as a nation. It supports our wider work to create a fairer and simpler NHS.”
According to the DHSC, steps are being taken to overcome potential disparities in the performance of medical devices. This includes the Medicines and Healthcare products Regulatory Agency (MHRA) requesting approval applications for new medical devices to include plans for addressing bias. Additionally, NHS guidance has been updated to highlight the potential limitations of pulse oximeter devices on patients with darker skin tones. Furthermore, the National Institute for Health Research (NIHR) is inviting funding applications for research aimed at developing smarter oximeters.
Furthermore, the government will work with the MHRA to ensure regulations for medical devices are safe for patients, regardless of their background, while allowing the introduction of more innovative products into the UK market. This includes ensuring the safety and effectiveness of pulse oximeters for all patients, with ongoing efforts to mitigate any inaccuracy in the devices.
Additionally, the DHSC will be driving forward initiatives to remove racial bias in datasets for clinical studies, upskill clinical professionals on issues including health equity, and improve transparency of data used in the development of medical devices using Artificial Intelligence (AI).
Professor Whitehead, chair of the review, acknowledged that while the advance of AI in medical devices could bring great benefits, it could also bring harm through “inherent bias against certain groups in the population, notably women, people from ethnic minorities and disadvantaged socio-economic groups.”
The review revealed how existing biases and injustices in society can unknowingly be incorporated at every stage of the lifecycle of AI-enabled medical devices, and subsequently amplify in algorithm development and machine learning.
Therefore, the health experts called for system-wide action, requiring full government support.
“The UK would take the lead internationally if it incorporated equity in AI-enabled medical devices into its Global AI Safety initiatives,” Professor Whitehead added.
Additionally, the review recommended ways of developing bias-free medical devices in the future and improving standards globally.
Dr June Raine, MHRA chief executive, acknowledged that inequities can exist within medical devices and welcomed the publication of the independent review.
“We are highly committed to ensuring equitable access to safe, effective and high-quality medical devices for all individuals, and the recommendations set out in this report will support and strengthen the impact of our ongoing work in this area.
“We are committed to working collaboratively with government, regulatory bodies, healthcare professionals and stakeholders to address these issues effectively,” she said.