Quadrant Pharmaceuticals Ltd has reported an error with the Patient Information Leaflets (PILs) that have been packed in certain batches of the products.
The Medicines and Health products Regulatory Agency (MHRA) on Thursday issued a class 4 medicines defect information notice for Cozaar 100mg film-coated tablets due to an error in the Patient Information Leaflets (PILs) reported by the company.
Cozaar is used to treat high blood pressure (hypertension) as well as to slow the progression of kidney disease in people who have type 2 diabetes mellitus.
Quadrant Pharmaceuticals Ltd has informed the UK regulatory authority that the PILs that have been packed in certain batches of the products do not have the most up to date safety information.
‘Grapefruit juice should be avoided while taking Cozaar’- This information is missing in Section 2 ‘What you need to know before you take Cozaar’, sub section ‘Cozaar with food and drink’, the company clarified.
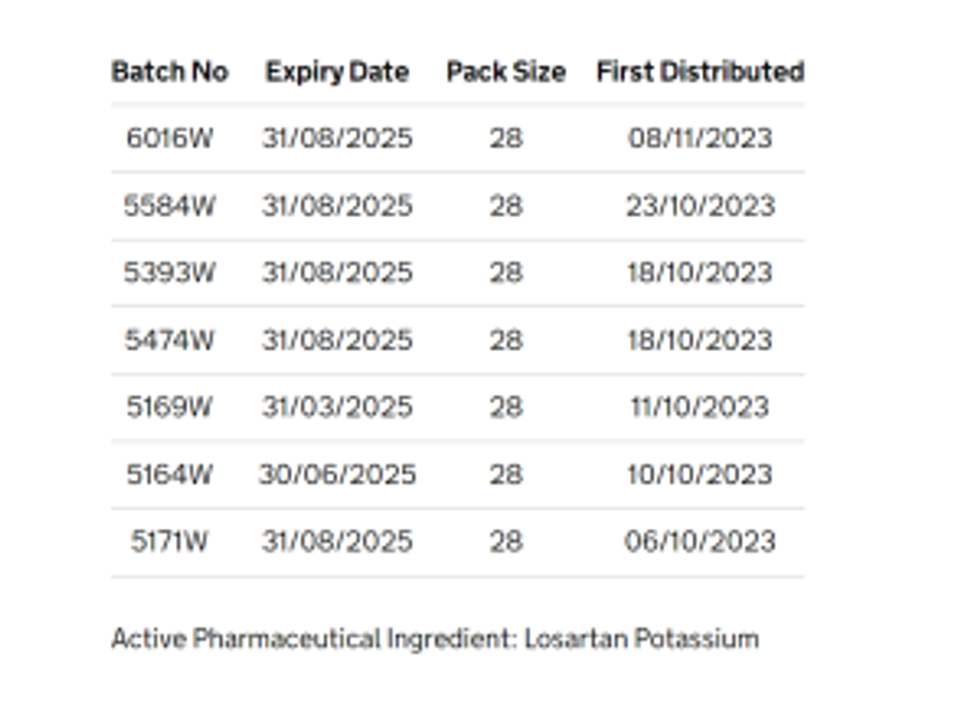
The company has listed the affected batches, but they are not being recalled as “there is no risk to product quality because of this issue.”
Patients are advised to continue to take the medicine as prescribed by their healthcare professional, and avoid drinking grapefruit juice while taking it.
Healthcare professionals have been directed to provide an updated PIL, where possible, when dispensing the tablets, or advise patients of the missing information.
Quadrant Pharmaceuticals Ltd has confirmed that all future imported batches will contain the correct PIL, and is ready to send hard copies of the updated PIL to wholesalers and pharmacies, upon request, so that “any remaining stock in the dispensary can be supplemented with the correct PIL information.”