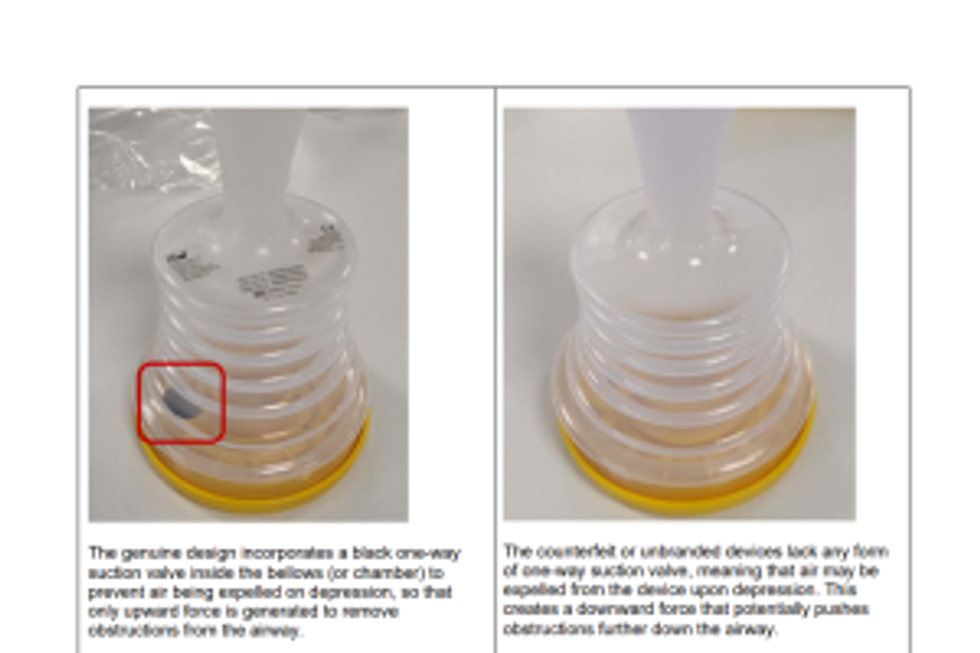
People are warned that use of unbranded devices could exacerbate the situation by pushing obstructions further down into the airway passage
The Medicines and Healthcare products Regulatory Agency (MHRA) has advised the public to exercise caution when buying anti-choking devices online, ensuring these products are purchased from reputable sellers.
As estimated by the regulator, more than 10,000 counterfeit or unbranded anti-choking devices have been purchased by the public within the last two years through listings on online marketplaces such as Amazon and eBay, as well as drop-shipping websites.
People are cautioned that the use of such products poses a substantial risk of failure in clearing blockages and could exacerbate the situation by pushing obstructions further down into the airway passage.
Dr Alison Cave, MHRA Chief Safety Officer, said: “Buying anti-choking devices that do not have a valid UKCA or CE mark increases the risk of receiving a product which does not include appropriate instructions and is either fake or does not meet the UK’s regulatory requirements.
“These products do not meet our strict quality standards and may put your health and safety at significant risk by failing to resolve or even worsening choking incidents.”
Currently, two anti-choking device brands, LifeVac and Dechoker, have a valid UKCA or CE mark and are authorised by the MHRA to be used after Basic Life Support protocols have been attempted and proved ineffective.
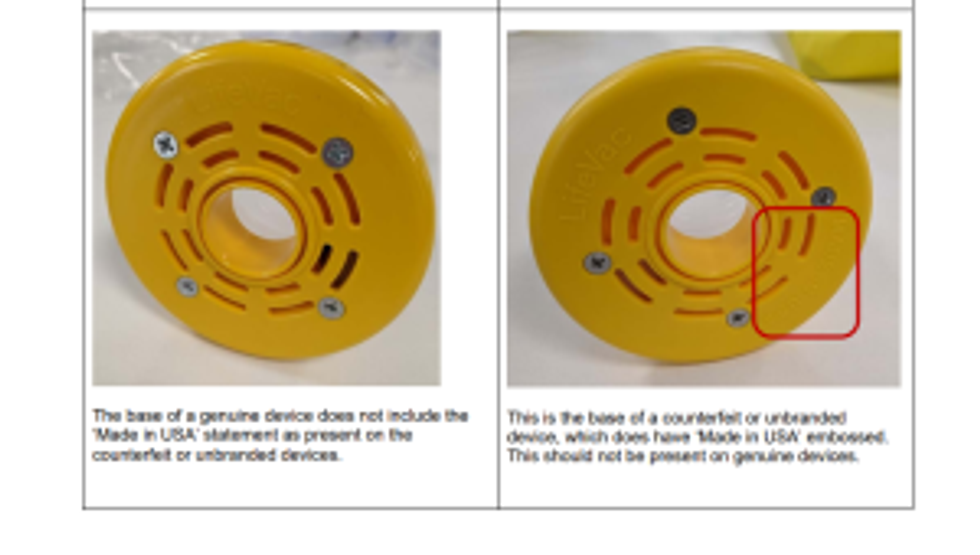
According to the regulator, most counterfeit or unbranded anti-choking devices are manufactured or shipped from China. These fake products resemble the design of the LifeVac device and, in some cases, claim to be the genuine brand.
“None of the anti-choking devices registered with the MHRA are manufactured in China. Any products shipped from China should be treated with caution and disposed,” the MHRA warning said.
How to check whether the device is genuine or counterfeit?
Photos highlighting the differences between a genuine and a counterfeit anti-choking device are provided in the Device Safety Information alert released by the MHRA
Key distinctions between a genuine and counterfeit device are highlighted here.
Buyers can also contact the legal manufacturer, LifeVac, which can verify the legitimacy of a purchased product against their records of authorised distributors and product serial numbers.
However, the MHRA has not received any reports regarding adverse incidents related to anti-choking devices.