In addition to significantly reducing treatment time for patients, the new multiple sclerosis injection could also save time for clinicians
Multiple sclerosis
Great news for multiple sclerosis patients! The NHS has introduced a new 10-minute injection that can slow the progression of disability while reducing hospital treatment time by over 90 per cent.
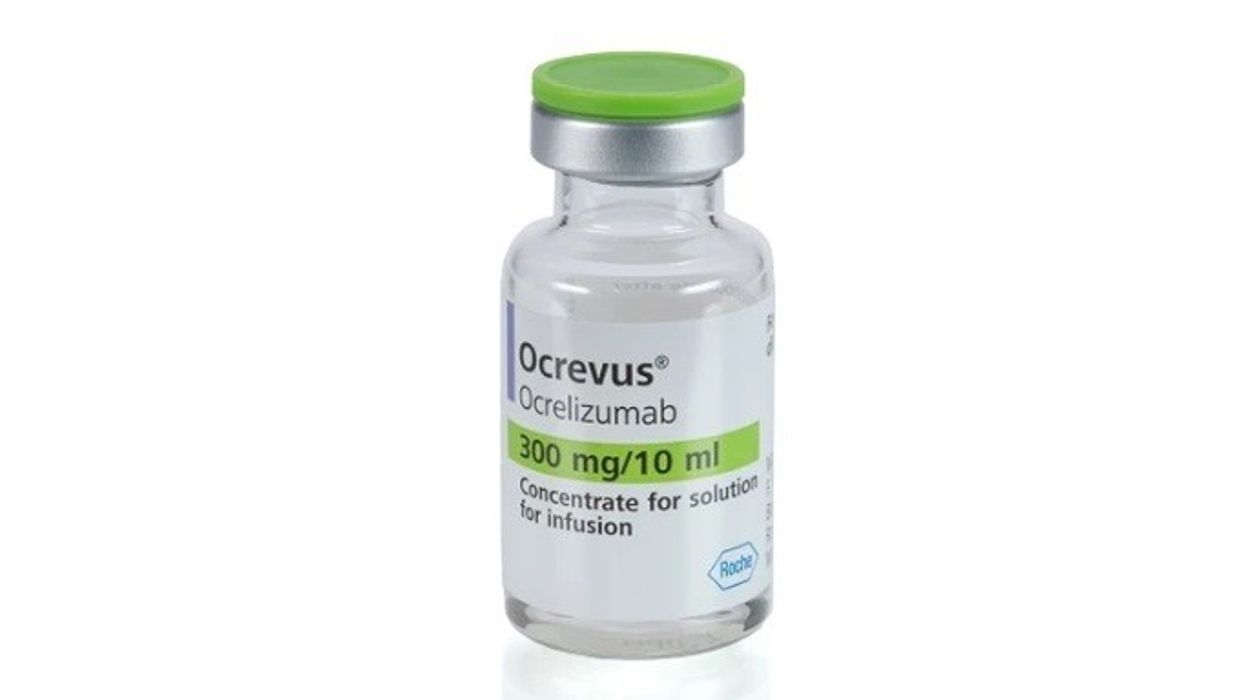
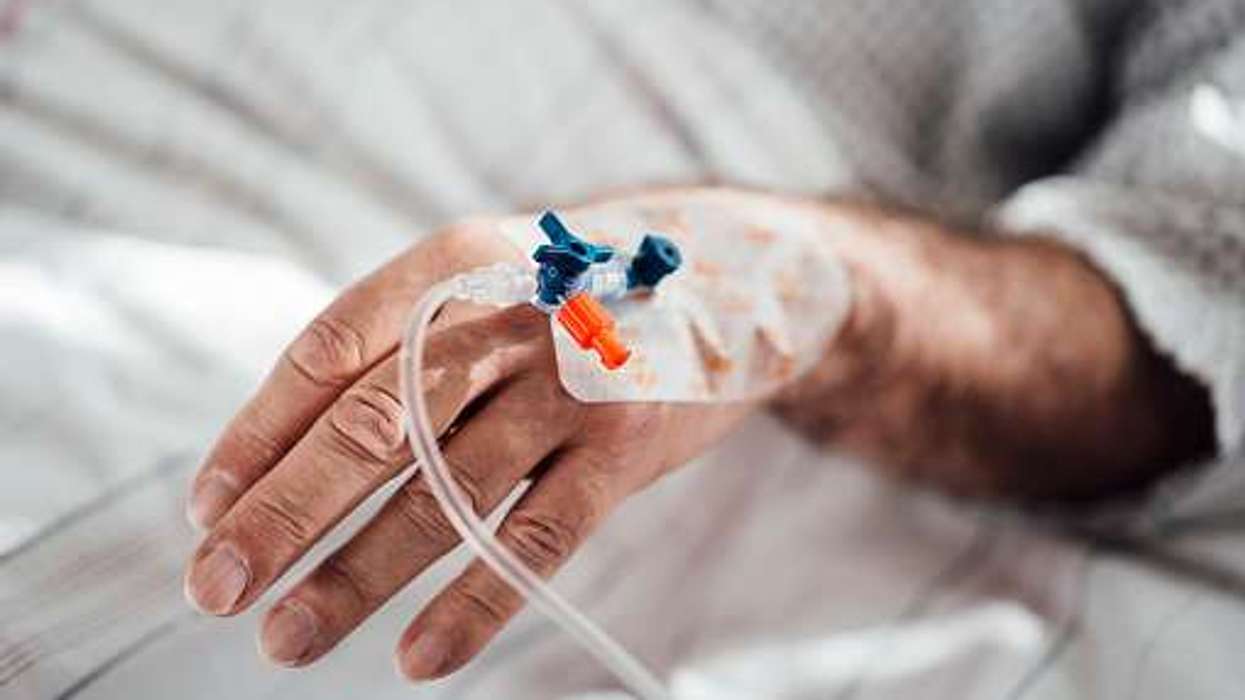
Currently, MS patients in England receive ocrelizumab (Ocrevus), manufactured by Roche, through twice-yearly intravenous (IV) infusions that can last up to four hours.
Now, around 9,000 NHS patients in England will be able to receive the drug via a quick ‘under-the-skin’ twice-yearly injection. It takes just ten minutes, enabling patients to spend less time in the hospital receiving treatment.
This roll-out follows approval from the Medicines and Healthcare products Regulatory Agency (MHRA), making the NHS one of the first healthcare systems worldwide to offer this new MS injection. Drug stocks are expected to be available in the coming weeks.
Clinical trials have shown the injection is as effective as the IV treatment, with 97 per cent of patients experiencing no relapses or brain lesion development over 48 weeks.
Professor Sir Stephen Powis, NHS national medical director, said: “This new injection will drastically cut the time that regular treatment takes for those living with multiple sclerosis, meaning that thousands of patients can spend less time in hospital while helping free up clinicians’ time to see more patients as well as vital capacity on wards.
“Ocrelizumab has represented a huge advance in care in recent years as the first drug able to change the course of the disease, and we hope this innovative and speedier option will now make another significant difference in improving patients’ quality of life and help thousands avoid longer stints in hospital for treatment.”
Ocrelizumab, a disease-modifying therapy for active relapsing or primary progressive multiple sclerosis, targets a specific type of immune cell to effectively halt MS symptoms.
Nin Sambhi, a 39-year-old MS patient from Staffordshire who currently takes ocrelizumab via infusion, is excited about the new injection.
“Ocrelizumab is working well for me right now and making me hopeful for a better and healthier future, but to be able to have an injection would be so much more convenient for me,” said Nin, who was diagnosed with relapsing MS in 2022.
“This new treatment would mean significantly less time spent in hospital and more time with my family,” added the mother of two young children.
Ceri Smith, Head of Policy at the MS Society, stated that this method will expand treatment choices for many MS patients, allowing them to receive treatment in a way that suits them best.
Multiple sclerosis, a lifelong condition affecting over 150,000 people in the UK, causes a range of symptoms including vision, mobility, and balance issues, and can sometimes lead to serious disability.
Multiple sclerosis is estimated to affect more than 150,000 people in the UK, including over 120,000 people in England. Each week around 135 people in the UK are diagnosed with this lifelong condition, which is known to be more common in women.
It can affect the brain and spinal cord, causing a range of symptoms including vision, mobility, and balance issues, and can sometimes lead to serious disability.
There are three main types of MS. Around 85 per cent of those with MS have relapsing-remitting MS, experiencing episodic attacks of symptoms Taking disease-modifying therapy can help them reduce relapses and slow progression.
About 10-15 per cent of patients have primary progressive multiple sclerosis, where symptoms worsen over several years without remission.
Secondary progressive MS follows relapsing-remitting MS but it can be delayed longer with disease-modifying therapies.
Ocrelizumab was first approved by the National Institute for Health and Care Excellence (NICE) for relapsing remitting multiple sclerosis in 2018, and for primary progression MS in 2019.
Oliver Fairweather, UK Lead for Multiple Sclerosis, Roche Products Limited said: “We are pleased that eligible NHS patients across England will have access to the recently licensed ocrelizumab subcutaneous injection.
“This means that those eligible patients living with both relapsing and early primary progressive multiple sclerosis are gaining access to a subcutaneous formulation of an established disease modifying therapy.”