Key Summary
- National Institute for Health and Care Excellence has recommended the first-ever disease-modifying treatment for ultra-rare ARG1 deficiency.
- Pegzilarginase (Loargys) replaces a missing enzyme, helping lower toxic arginine levels and improve movement and thinking.
- Around 20 people in England could now access this treatment on the NHS, offering new hope for patients and families.
The National Institute for Health and Care Excellence (NICE) has recommended the first-ever disease modifying therapy, pegzilarginase, for arginase 1 deficiency (ARG1-D), an ultra-rare health condition.
ARG1-D is an inherited and progressive metabolic disorder that prevents the production of the enzyme arginase in the body.
The enzyme is necessary to remove toxic wastes such as ammonia via the process of urination and avoid serious neurological conditions.
Without a cure or disease modifying treatment for ARG1-D, health experts focus on managing symptoms through protein restricted diets, proper feeding intervals, and medicines to control the amount of ammonia in the patients’ blood.
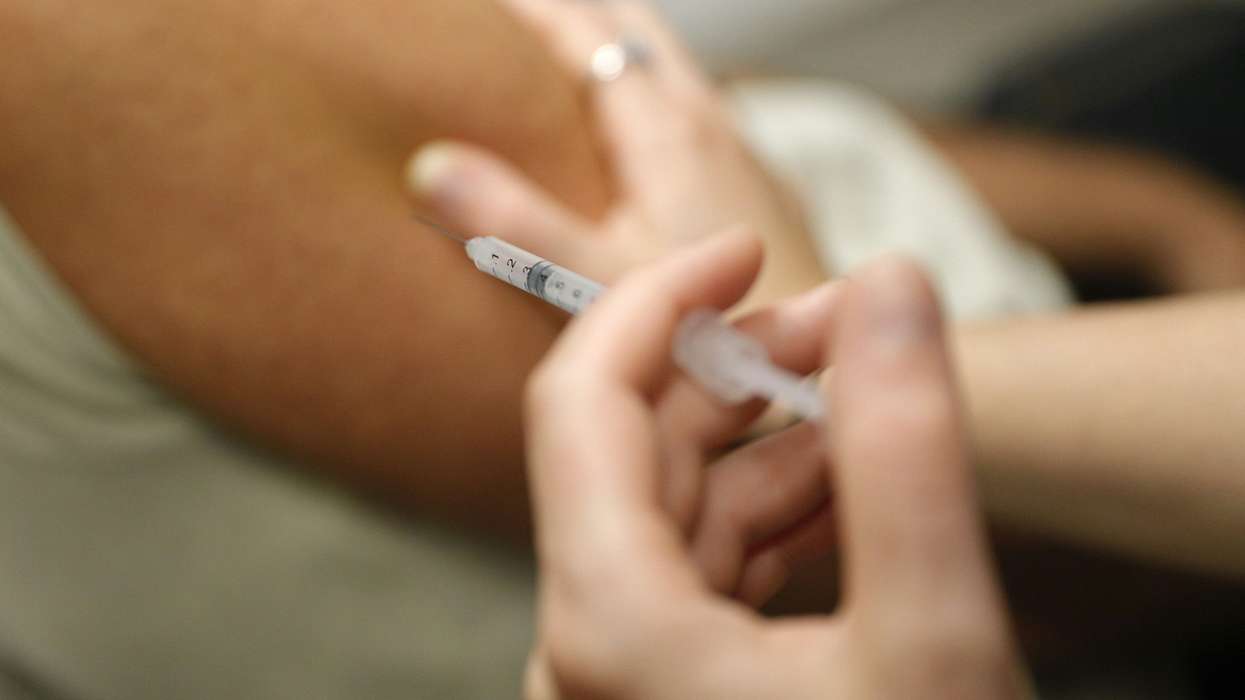
Pegzilarginase, also known as Loargys, developed by Immedica, is now recommended for adults, adolescents and children aged 2 years and older via NHS England.
It is an enzyme replacement therapy given in the form of a drip or an injection.
Learning difficulties, stiff muscles, seizures and slow growth are some of the common symptoms of the condition.
The clinical trials revealed that the introduction of pegzilarginase reduced the amount of arginine in the patients’ blood and improved their mobility and mental processing.
It is the 32nd treatment approved by NICE out of the 34 treatments under their Highly Specialised Technologies programme for treatments for ultra-rare diseases since 2015.
"It is estimated that 20 people in England have ARG1-D and today’s decision means pegzilarginase will give some of them and their families real hope of a better quality of life,” commented Helen Knight, director of medicines evaluation at NICE.
Jack Turner, deputy director of medicines negotiation & access at NHS England added, “It’s fantastic news for patients and their loved ones that this pioneering, first-in-class treatment will now be available on the NHS to help manage some of the complex, challenging symptoms of this ultra-rare condition.”