Key Summary:
- Teplizumab (Tzield) becomes the UK’s first approved immunotherapy for type 1 diabetes
- Delays progression from Stage 2 to Stage 3 T1D by about three years in patients aged 8+
- 14-day daily IV infusion; safety and effectiveness will be continuously monitored
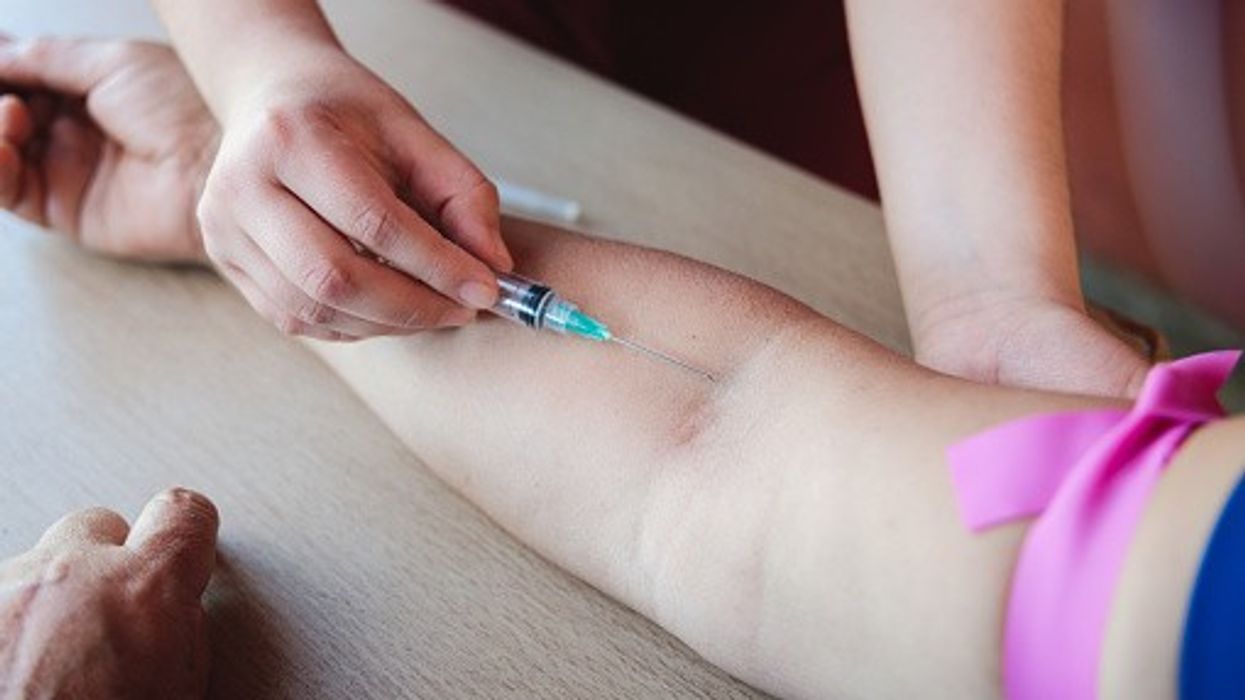
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved Teplizumab, an immunotherapy for type 1 diabetes.
Teplizumab (Tzield) helps delay the onset of Stage 3 type 1 diabetes (T1D) by an average of three years in adults and children aged eight years and older with Stage 2 T1D.
Teplizumab is administered through intravenous infusion – once in a day for 14 days.
This is the UK’s first-ever approved immunotherapy to treat type 1 diabetes, and this medicine has been approved through the International Recognition Procedure (IRP), which allows the MHRA to take into account the expertise and decision-making of trusted regulatory partners for the benefit of UK patients.
During the Stage 3 type 1 diabetes, patients begin to experience blood sugar problems and are diagnosed with the condition, which often requires lifelong insulin treatment.
Teplizumab is used in people with Stage 2 T1D, which is an earlier stage of the disease where individuals are at high risk of progressing to Stage 3.
To ensure patient safety, the MHRA will continue to review the safety and effectiveness of teplizumab.