Healthcare professionals have been told to stop supplying two contaminated batches of paracetamol ‘immediately’ and quarantine all remaining stock before returning it to their supplier.
The Medicines and Healthcare products Regulatory Agency (MHRA), issued the warning after the discovery of a few ‘discoloured’ tablets.
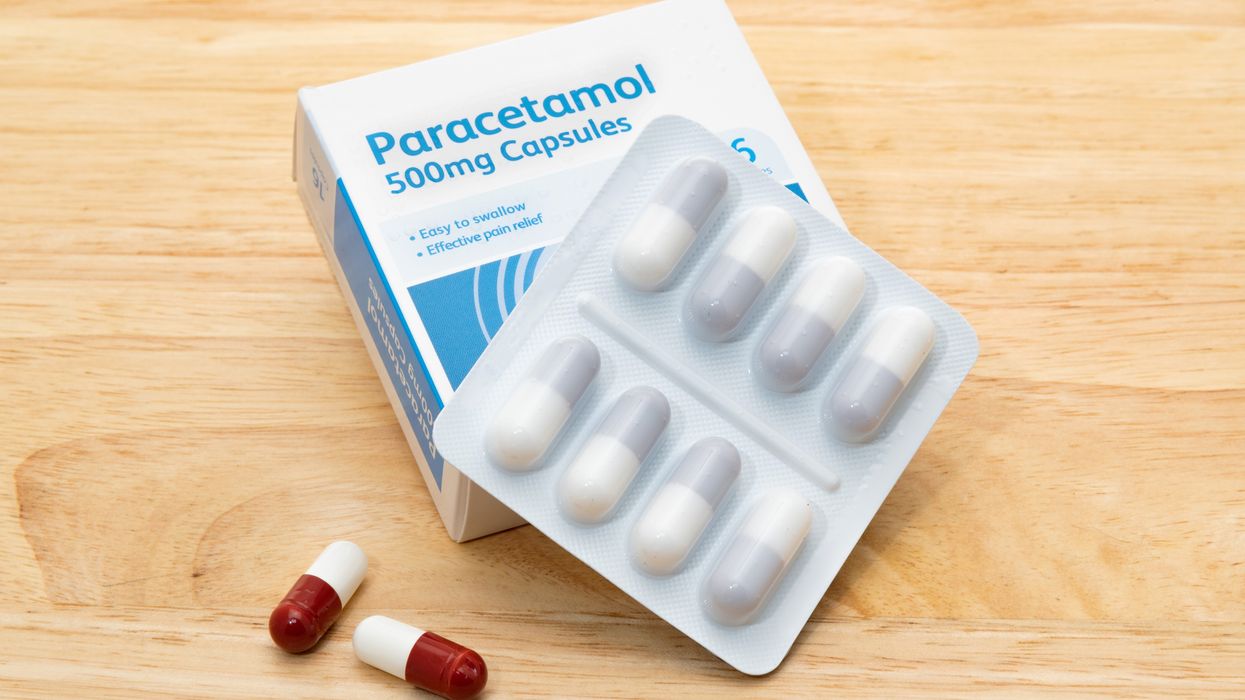
The precautionary recall was made for two batches of paracetamol 500mg pills manufactured by Chelonia Healthcare Limited.
Patients have been advised to check the packaging to see if they have purchased the pills from any batches with codes 2312010 and 2312011 with the expiry dates of the 30th and 31st of November, 2027.
The recall notice points out that the tablets should be white capsule shaped and scored on one side.
The previous cases of discoloration in paracetamol tablets were related to fungal contamination.
“If you find tablets that are discoloured in any way, in pots from the listed batches, please contact your pharmacist or the healthcare professional who dispensed your prescription,” stated the recall notice.
Patients who have unknowingly consumed the pills and experience ‘adverse reaction’ are advised to seek medical help.
The MHRA also said that the specific batches of this medicine can only be bought at a pharmacy with a prescription.
Similar incidents have taken place across the world. Europe experienced a similar recall in 2019 as a few batches of medicines there were contaminated with a strain named Penicillium citrinum.
According to studies, paracetamol is one of the most widely used medications in the UK, where approximately 6,500 tonnes of it are sold in Britain annually, which is 70 pills per person in a year.
Data shows that more than a million prescriptions of paracetamol tablets are made by the NHS in England monthly, costing more than £5million.