The GPhC, the Advertising Standards Authority (ASA) and the Medicines and Healthcare products Regulatory Agency (MHRA) have issued an updated joint notice making clear that adverts for named prescription-only medicines for weight-management are prohibited.
The three regulatory organisations are working together to remind advertisers that action will be taken to tackle ads that violate the rule.
The Enforcement Notice was first issued and sent to pharmacy owners, pharmacists and pharmacy technicians in April 2025.
It reminds advertisers that advertising prescription-only medicines, including those used for weight management, is banned.
It also sets out the enforcement action the agencies will take against erring advertisers and businesses.
The updated Enforcement Notice follows nine ASA rulings published in July, which gave clear examples of where, even without naming specific prescription-only medicines, ads were still seen as promoting weight-loss prescription-only medicines.
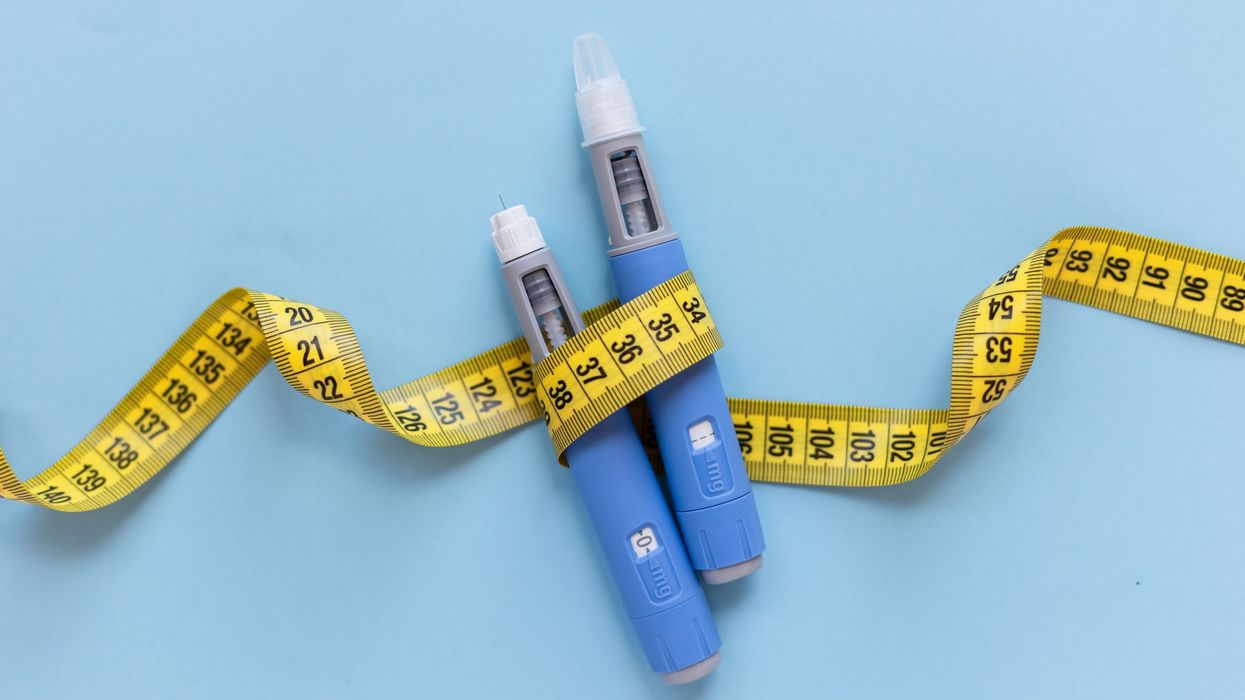
The notice says that advertisers should avoid words and expressions such as “weight-loss injection”, “obesity treatment jab” or “GLP-1”.
They should also avoid imagery that is likely to suggest an injection pen.
The advertisers should also avoid ads for general weight management products or services that direct consumers to other ads, such as website landing pages, promoting POMs.