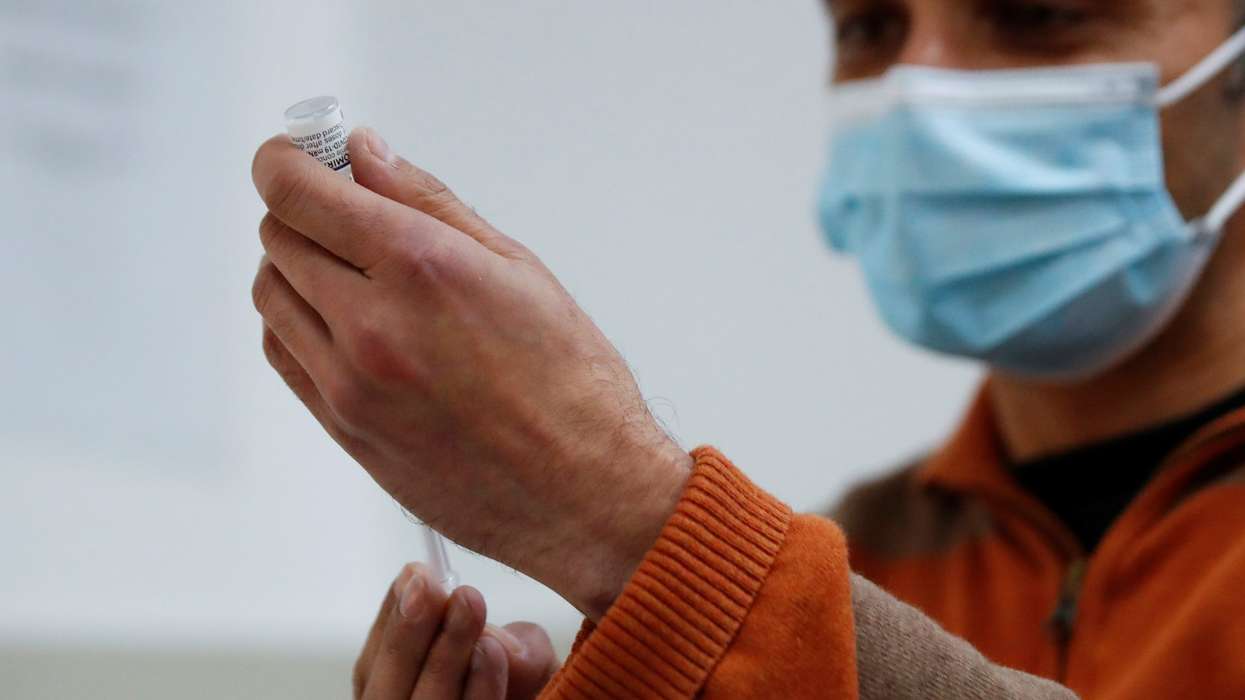
The Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for Vimkunya, a vaccine developed by Bavarian Nordic A/S to prevent chikungunya disease in people aged 12 years and older.
The UK approval follows earlier approvals by the U.S. Food and Drug Administration (FDA) and the European Commission in February 2025, making this the third regulatory green light for the vaccine.
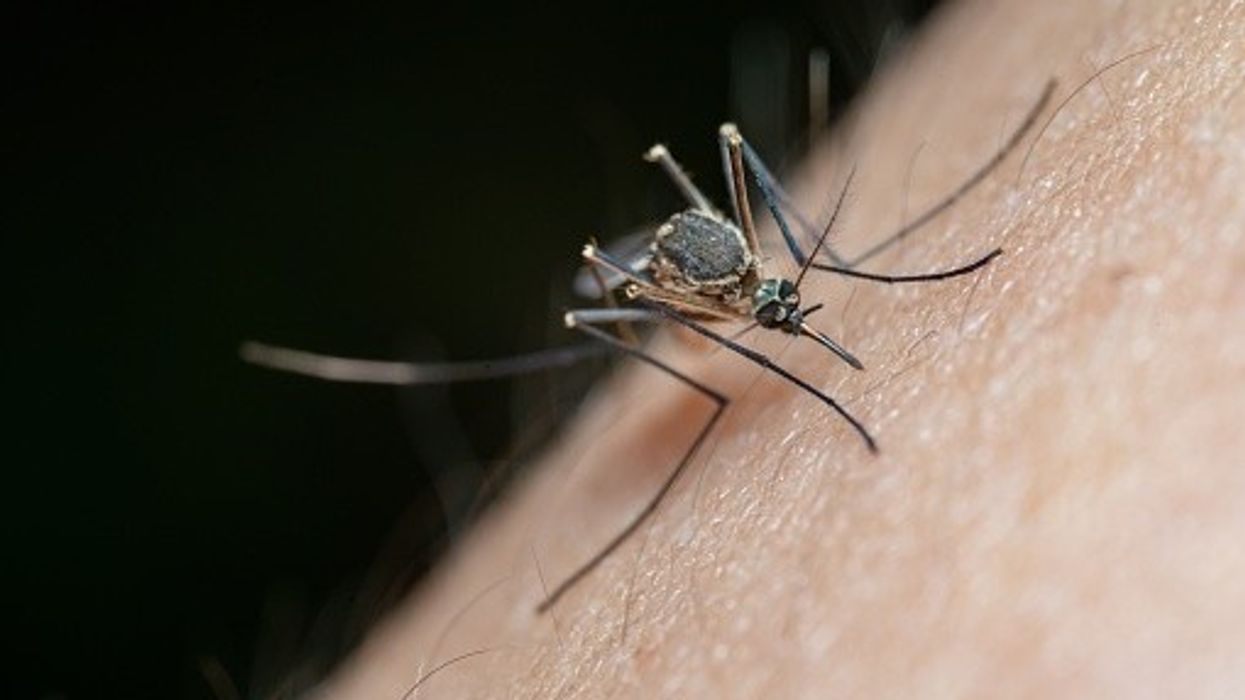
Chikungunya is a mosquito-borne disease caused by the chikungunya virus (CHIKV), which is found in countries across Asia, Africa and the subtropical regions of the Americas.
Most people infected with the virus develop fever, rash, and severe pain in multiple joints that typically resolve between one to two weeks, but symptoms may last for months or years in some cases.
“Chikungunya mostly represents a risk for UK citizens traveling overseas to affected regions in the Americas, Africa and Asia, but as recent research has shown, invasive mosquitos known to carry the disease have established themselves in many parts of Southern Europe and are moving further north due to climate change,” said Paul Chaplin, President and CEO of Bavarian Nordic.
“The mosquitos cannot be stopped, but with preventative measures such as vaccines, we can mitigate the impact of emerging diseases like chikungunya for those at risk.”
Vimkunya is a single-dose, prefilled, adjuvanted virus-like particle (VLP) recombinant protein vaccine designed for active immunization against chikungunya virus (CHIKV) in individuals aged 12 and above.
The MHRA approved the vaccine through the International Recognition Procedure (IRP), which allows streamlined assessment by recognising prior approvals from trusted international regulators—in this case, the European Commission.
The approval was based on results from two phase 3 clinical trials, which showed that 21 days after a single dose, the vaccine induced neutralising antibodies in up to 97.8% of recipients aged 12–64 and 87.3% in those over 65.
Vimkunya was well tolerated, with the most common side effects being injection site pain, fatigue, headache, and muscle pain—all typically mild or moderate.
The Danish company plans to launch the vaccine in the UK in summer 2025, and has also submitted an application to Health Canada, with potential approval anticipated in the first half of 2026.