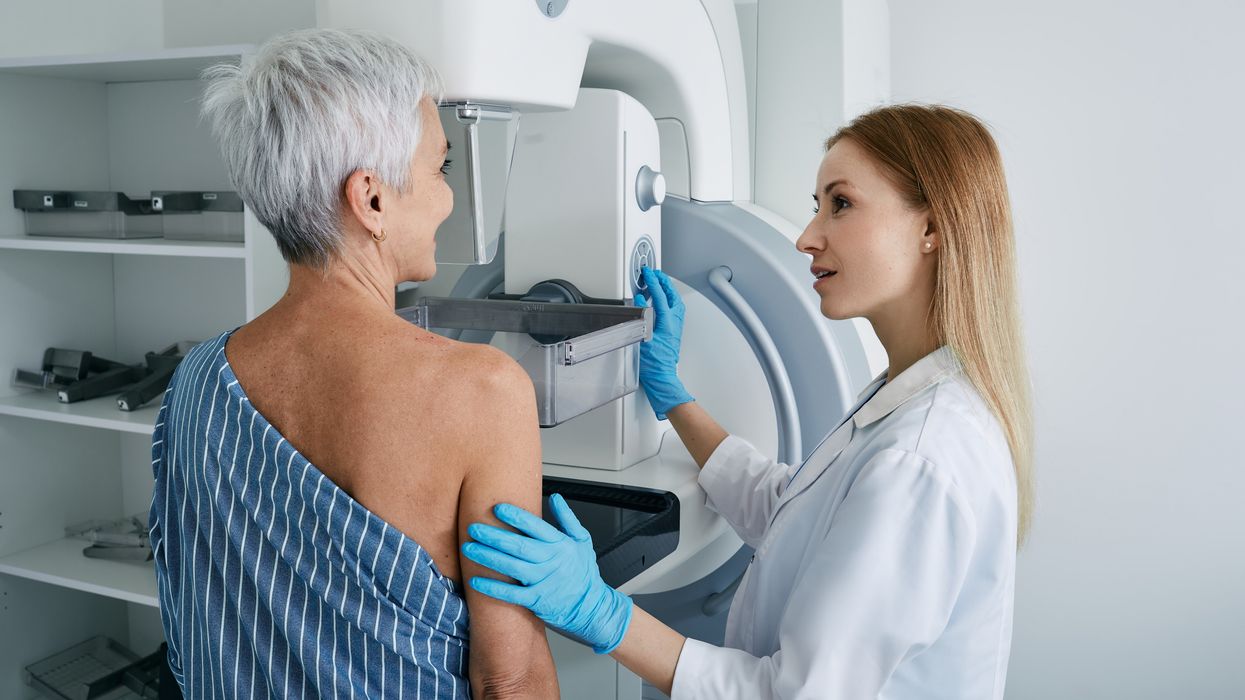
The hot flashes and night sweats that plague breast cancer survivors during years-long hormone-suppressing therapy are eased by an experimental non-hormonal drug being developed by Bayer, according to results from a late-stage trial.
The participants in the trial – similar to two-thirds of breast cancer patients overall - had tumors that use the hormones estrogen and progesterone to grow.
The goal of so-called endocrine therapy is to block those hormones, which reproduces the uncomfortable menopause symptoms. The most effective way to relieve these symptoms in healthy women is to replace the hormones, which is not feasible when tumors use the hormones to grow.
In a year-long trial involving 474 breast cancer patients experiencing daily hot flashes due to hormone-suppressing therapy, 316 received Bayer’s elinzanetant and 158 received a placebo.
Within three months, more than 70 per cent of those on elinzanetant reported at least a 50 per cent reduction in moderate-to-severe hot flashes, compared to about 36 per cent of the placebo group, the researchers reported at a recent meeting of cancer doctors and in The New England Journal of Medicine.
The Bayer drug also significantly improved sleep quality and menopausal quality of life by week 12.
“It is important to treat vasomotor symptoms because they can negatively impact quality of life and lead to women prematurely stopping their breast cancer treatment,” said study leader Dr. Fatima Cardoso of the Champalimaud Clinical Center in Lisbon.
Elinzanetant belongs to a new class of drugs called neurokinin receptor antagonists that target the neurobiological mechanisms in the brain involved in hot flashes and night sweats.
The US Food and Drug Administration recently approved an Astellas Pharma 4503.T drug from the class under the brand name Vezoah for easing symptoms of menopause. It is not approved for treating breast cancer patients, so that use would be off-label, the study authors noted. Doctors can prescribe any approved medicine as they see fit, but companies can only promote them for approved uses.
An editorial published with the study notes that up to 90 per cent of women with early breast cancer treated with endocrine therapy experience hot flashes and other vasomotor symptoms, which may impact their survival if the symptoms lead them to quit taking the medications.
In one large study of breast cancer survivors, half the participants reported non-adherence to endocrine therapy, the editorial says.
Bayer is awaiting approval of elinzanetant from the FDA and the European Medicines Agency.