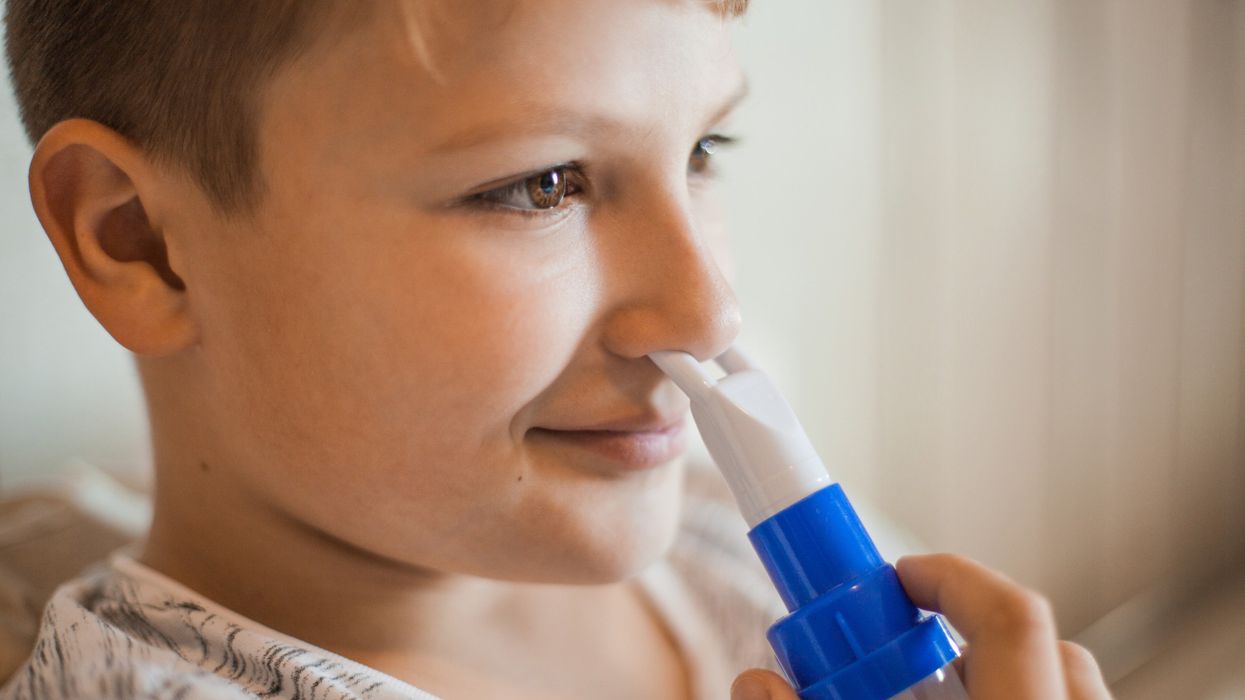
Children with diabetes who inhaled their mealtime doses of insulin did just as well as those who injected insulin under the skin, researchers reported at the American Diabetes Association scientific meeting in Chicago.
To regulate their blood sugar, patients with type 1 diabetes usually require an injection of a long-acting basal insulin once a day, plus additional injections of rapid-acting insulins at mealtimes.
MannKind's inhaled insulin Afrezza is approved for use by adults but not yet for children, which prompted the study.
The 230 children with type 1 diabetes, ages 4 to 17, who participated in the trial received either Afrezza at mealtimes, or their usual mealtime injections of insulin, for 26 weeks. Everyone continued to receive their basal insulin injections.
Control of hemoglobin A1c, a marker of blood sugar control over the past several months, was comparable with the inhaled insulin and the injected insulin, the researchers found.
Inhaled insulin was also associated with less weight gain and slightly higher child and parent preference scores.
The inhaled formula did not have any adverse effects on patients’ lungs, the researchers reported.
“Inhaled insulin is the fastest acting insulin available and is a valuable alternative to injected analogue insulin,” study leader Dr. Michael Haller of the University of Florida said in a statement. “Afrezza should be available as an option to all children and adults with type 1 diabetes.”
Eli Lilly's experimental once-weekly insulin efsitora was comparable to daily insulins in nearly a thousand adults with type 2 diabetes in three late-stage trials, researchers reported at the ADA meeting.
The trials, which were designed to study patients at different stages of insulin use, each found efsitora to be just as effective as daily insulins for bringing HbA1c levels - a common measure of blood sugar over time - under control.
“Once-weekly efsitora may offer a significant advancement for people with type 2 diabetes who need insulin by eliminating over 300 injections per year," Lilly’s senior vice president of product development, Jeff Emmick, said in a statement.
One trial, reported in The New England Journal of Medicine, involved patients with type 2 diabetes who were using insulin for the first time. A second trial in patients who had been using daily basal insulin degludec and a third trial in those who had been taking basal insulin glargine plus extra mealtime insulin doses were both reported in The Lancet.
Efsitora “has the potential to facilitate and simplify insulin therapy, reducing the hesitation often associated with starting insulin to treat type 2 diabetes," Dr. Julio Rosenstock of University of Texas Southwestern Medical Center, who led one of the studies, said in a statement.
People newly diagnosed with type 2 diabetes usually start treatment with oral medications, but roughly one-third of them will need to use insulin within 8 years of their diagnosis, according to an editorial in The Lancet.