Key Summary
- MHRA approves tofersen (Qalsody) for treating rare inherited ALS caused by SOD1 gene mutation
- Drug slows nerve damage by reducing toxic protein, given via spinal injection
- Common side effects include headache and fatigue; serious risks monitored through MHRA Yellow Card scheme
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved tofersen (Qalsody) to cure amyotrophic lateral sclerosis (ALS) due to the mutations in the SOD1 (an enzyme named superoxide dismutase 1) in adults.
This rare condition is an inherited type of motor neurone disease (MND).
ALS is a progressive disorder that impacts the nerve cells in the brain and spinal cord, weakening muscles leading to struggle in swallowing and even breathing.
Tofersen decreases the production of toxic protein by SOD1 gene that damages the nerve cells.
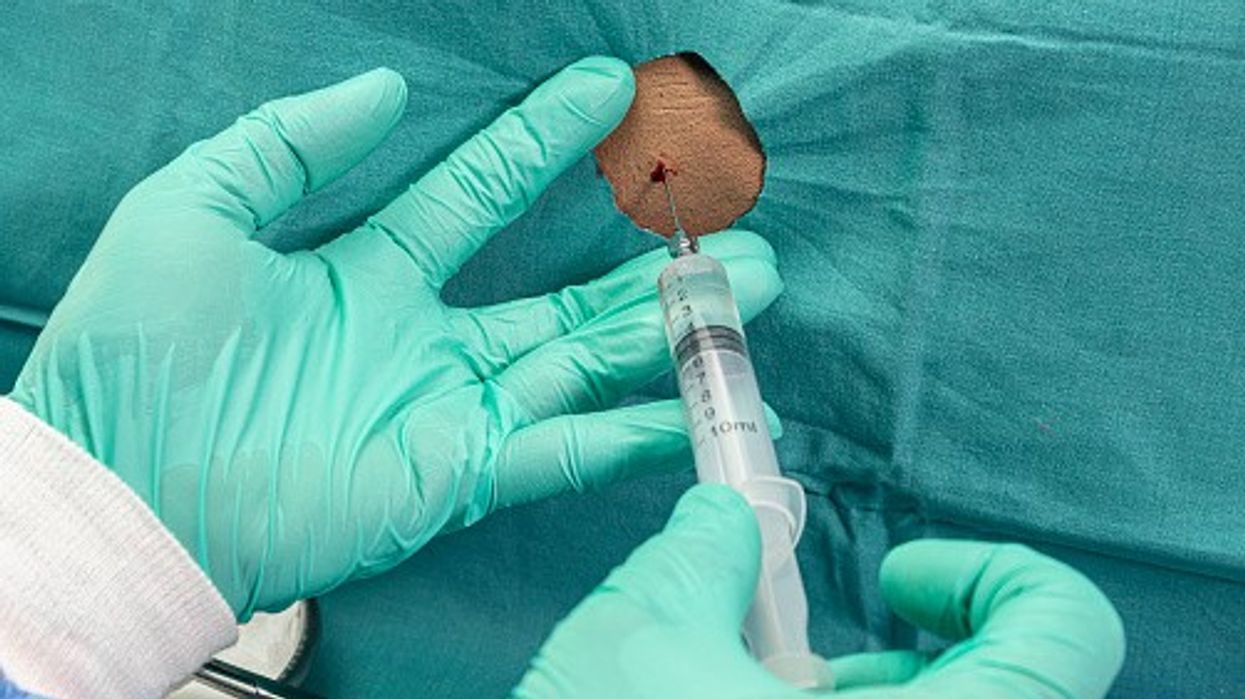
It is administered through lumbar puncture or injection into the lower spine at regular intervals by an experienced health professional to treat the disorder.
The MHRA has approved tofersen on July 22, via the International Recognition Procedure (IRP).
The common side effects of tofersen include headache, back pain and fatigue.
In rare cases it could lead to inflammation of the spinal cord or optic nerve, and increased pressure around the brain.
MHRA claims tofersen would also be under constant supervision for future improvements and for the prevention adverse incidents.
Anyone experiencing symptoms of side effects must immediately consult a doctor, pharmacist or nurse, along with the submission of a report to the MHRA Yellow Card scheme.