Retailers told to withdraw the affected products and collect them from the patients
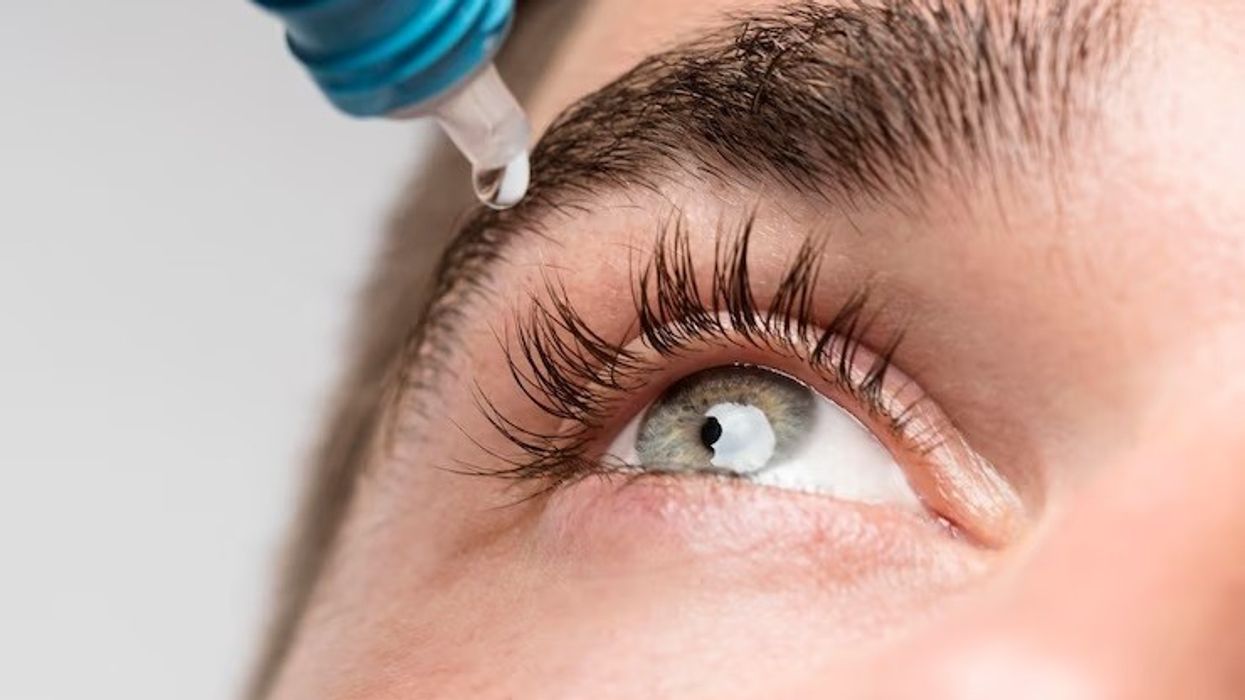
The Medicines and Healthcare products Regulatory Agency (MHRA) has cautioned people to stop using certain eye gels, highlighting a potential risk of microbial contamination that can cause an infection.
As a precaution, the agency on Friday announced recall of specific batches of carbomer-containing lubricating eye gels branded Aacarb, Aacomer and Puroptics, which are generally used to relieve the symptoms of dry eye.
Burkholderia cenocepacia is suspected to have caused the microbial contamination, and the issue was raised after an ongoing investigation conducted by UK Health Security Agency (UKHSA) identified a small number of cases of infection.
Investigations are on to determine if there is a link between these products and the infections which have been identified.
Meanwhile, retailers have been told to withdraw the affected products, and users are asked to return their product to the place of purchase immediately.
Alison Cave, MHRA Chief Safety Officer, said that the risk to users is low, but they are taking precautionary action.
“Retailers should, where possible, contact patients who have been dispensed any of the affected batches and ask them to return the product,” she said.
Further, Cave informed that they are working very closely with their colleagues at UKHSA and that further advice will be issued to protect patients and the public, if needed.
Those who have been using the recalled eye gels are asked to contact a healthcare professional if they’re feeling unwell with symptoms of eye infection, such as reduced vision, red and painful eye.
Individuals with cystic fibrosis and patients with certain risk factors are known to be at higher risk of adverse effects from the suspected bacteria.
Until further information is available, the UKHSA has recommended that all carbomer-containing lubricating eye gels are avoided, where possible, in individuals with cystic fibrosis, patients being cared for in critical care settings, those who are severely immunocompromised and in hospital, and patients awaiting lung transplantation.
The MHRA has urged healthcare professionals, or anyone who suspects they may have become unwell after using these eye gels, to report it the agency’s Yellow Card Scheme.